Rostrum Medical announces and is excited to share the publication of initial validation results of its novel VQm® System.
July 24, 2023 - Vancouver, B.C.
Management of mechanically ventilated patients can be complex due to the difficulty in directly assessing the impacts to the heart and lung following clinical interventions. Rostrum Medical, which has developed and designed the VQm® System to address these challenges in patient management and is excited to announce the publication of its initial clinical validation in a porcine model.
Rostrum Medical conducted the multicentre studies in 2020 at the Medical University of Vienna (MUV), Austria and Hôpital Européen Georges-Pompidou (HEGP) of Paris, France. The goal was to demonstrate the VQm® System, with its key parameters (Pulmonary Blood Flow, Functional Residual Capacity, and its’ novel Shunt Index) can achieve comparable measurements with current reference standards. By offering these parameters at the bedside, in near real time, non-invasively, the VQm could streamline the individualization of mechanical ventilation. The study found good agreement between the VQm® System and the reference standards, which supports the continued evaluation for application in intensive care units or operating rooms.
Since completing the animal study in 2020, Rostrum has successfully completed in-human studies at University California Davis, Sacramento while establishing multiple ongoing and planned studies at leading research centres in Canada, Europe, and the US. The VQm® System is currently approved for sale in Canada.
“We are very pleased to recognize the strong positive outcomes that this study produced. This will be the first of many publications that we will be announcing, and we are excited to continue to progress with our on-going evaluations” says Nathan Ayoubi, COO of Rostrum Medical.
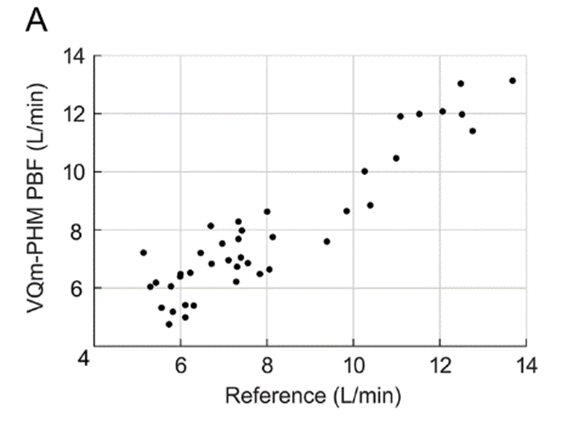
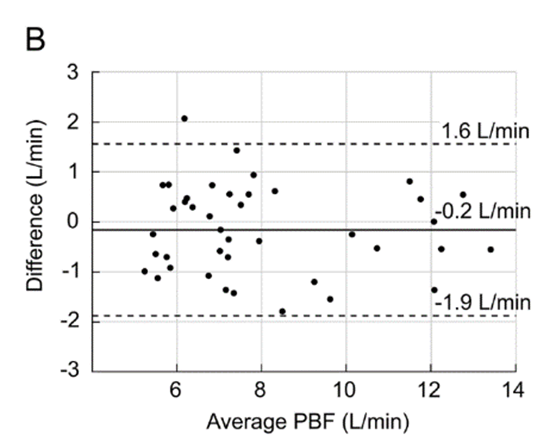
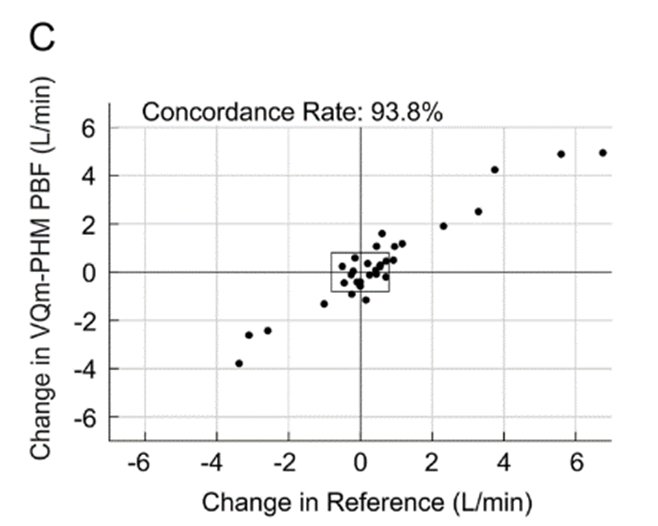
Figure 1: Pulmonary Blood Flow (PBF) measurement obtained by the VQm® was compared to the reference measurement (TDCO – Berggren Shunt). The 95% limits of agreement were 1.5 and 1.9L/min.
Figure 2: PEEP intervention was designed to demonstrate changes in FRC measured by the VQm®. The mean FRC during baseline and high PEEP were 1.7 ± 0.4 L and 2.0 ± 0.6 L, which resulted strong statistical evidence with p = 0.0078.
Figure 3: Shunt Index (Qsi) measurements obtained by the VQm was compared to the reference measure (Berggren Shunt). The mean Qsi during low and high shunt flow was 40 ± 4 and 27 ± 5, which resulted strong statistical evidence with p = 0.0078.
To read the full publication, click here.
About Rostrum Medical Innovations
Rostrum Medical Innovations is dedicated to empowering medical practitioners with new and useful solutions to help solve everyday challenges that medical professionals face. Rostrum was established with a goal to deliver novel solutions to problematic uncertainties in modern day practice. With a team of some of the world’s most progressive clinicians, combined with engineering and business expertise with a history of commercial success, Rostrum is driven by one mission – to address clinical needs in healthcare.
About VQm Pulmonary Health Monitor™
The VQm® is the first system to provide direct simultaneous heart and lung measurements to allow for earlier trend identification and individualized management of mechanically ventilated patients. The VQm® is the first non-invasive, bed-side system to enable clinicians to easily assess the impacts of their clinical interventions of mechanically ventilated patients in near real-time, optimizing clinical workflow, personalizing care for potential improvements in patient outcomes.
For more information or inquiries, please email: media@rostrummedical.com